Safety Notice - Sleep Therapy Masks with Magnets
Philips Patient Hotline:
877-387-3311
8:30am - 5pm ET
Montday - Friday
- FDA Safety Notice concerning Philips PAP masks with magnets
- Philips Respironics alerts customers worldwide of updated instructions and labeling of specific sleep therapy masks that contain magnetic headgear clips due to potential risk of serious injury
- Philips Respironics FAQ - Notification regarding masks with magnets
- Philips Respironics - Notification to Patients
Philips Respironics has distributed the notice below, though magnets may be used in other manufacturers' masks. Patients should check with their medical equipment supplier to be sure.
Philips Respironics is alerting customers worldwide of updated instructions and labeling of specific sleep therapy masks that contain magnetic headgear clips due to potential risk of serious injury.
The following Philips Respironics patient interface devices (face and nasal masks) contain magnets - Amara View Minimal Contact Full-Face Mask, DreamWear Full Face Mask, DreamWisp Nasal Mask with Over the Nose Cushion, Wisp Nasal Masks, Wisp Youth Nasal Masks, and Therapy Mask 3100 NC/SP.
This Urgent Medical Device Correction is intended to inform you that Philips Respironics is updating its existing 'Contraindications' and 'Warning' of the above masks with magnets to the below.
Contraindication: Use of the mask is contraindicated for patients and their household members, caregivers, and bed partners that may be in close vicinity to patients using the masks, that have implanted devices that may be affected by magnets, including but not limited to:
- Pacemakers
- Implantable cardioverter defibrillators (ICD)
- Neurostimulators
- Magnetic metallic implants/electrodes/valves placed in upper limbs, torso, or higher (i.e. neck and head)
- CSF (cerebral spinal fluid) shunts (e.g., VP (ventriculo peritoneal) shunt)
- Aneurysm clips Embolic coils
- Intracranial aneurysm intravascular flow disruption devices
- Metallic cranial plates, screws, burr hole covers, and bone substitute devices
- Metallic splinters in the eye
- Ocular implants (e.g., glaucoma implants, retinal implants)
- Certain contact lenses with metal
- Implants to restore hearing or balance that have an implanted magnet (such as cochlear implants, implanted bone conduction hearing devices, and auditory brainstem implants)
- Magnetic denture attachments
- Metallic gastrointestinal clips
- Metallic stents (e.g., aneurysm, coronary, tracheobronchial, biliary)
- Implantable ports and pumps (e.g., insulin pumps)
- Hypoglossal Nerve Stimulators
- Devices labeled as MR (magnetic resonance) unsafe
- Magnetic metallic implants not labeled for MR or not evaluated for safety in a magnetic field
Warning: Magnets with a magnetic field strength of 400 mT are used in the mask. With the exception of the devices identified in the contraindication, ensure the mask is kept at least 6 inches (approx. 15.24 cm) away from any other medical implants or medical devices that can be impacted by the magnetic fields to avoid possible effects from localized magnetic fields. This includes household members, caregivers, and bed partners that may be in close vicinity to patients that use the masks.
- Patients should STOP using the affected mask, if the implant/medical device is contraindicated against the mask magnets. Patients should consult their physician immediately to determine if another mask can be used for their therapy. In the interim, switch to a non-magnetic mask if available, for continued therapy. Patients should properly dispose of the mask that has magnets after an alternative is obtained.
- If patients, and household members, caregivers, and bed partners that may be in close vicinity to patients, do not have implanted medical devices, or metallic splinters in their eyes, then no action related to patients is needed.
- Contact Philips Respironics customer service to learn more about non-magnetic mask options.
- Household members, caregivers, and bed partners with a medical implant/device must ensure the mask is kept at least 6 inches (approx. 15.24 cm) away from the medical implant(s)/device(s).
-
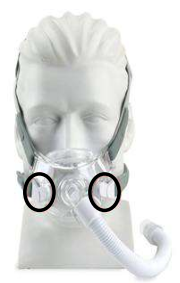
Amara View Full
Face Mask -
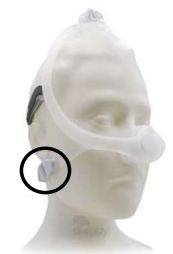
DreamWisp
Nasal Mask -
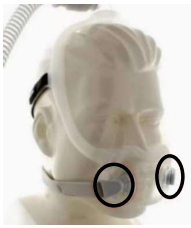
DreamWear Full
Face Mask -
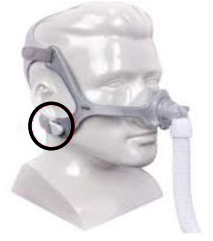
Wisp and
Wisp Youth
Nasal Mask -
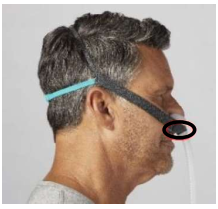
Therapy Mask
3100 NC/SP
Back to top
